



















Which species is the conjugate base of NH3. NH2-Correctly identify the correct conjugate acid-base pairs in the flowing equation: HC2H3O2(I) + H2O(I) ⇌ H30^3(aq) + C2H3O2-(aq) Quizlet Live. Quizlet Learn. Diagrams. Flashcards. Mobile. Help. Sign up. Help Center. Honor Code. Community Guidelines. Answer to 3.) Give the formula for the conjugate base of each species: (a) H2S; (b) HCN; (c) HSO4−. (a) (b) (c)... The Arrhenius definition of acid and base is limited to aqueous (that is, water) solutions. Although this is useful because water is a common solvent, it is limited to the relationship between the H + ion and the OH − ion. What would be useful is a more general definition that would be more applicable to other chemical reactions and, importantly, independent of H 2 O. The amide ion, NH_2^-. The conjugate base of any species is that species less a proton. Ammonia less a proton is the amide ion, NH_2^-. Mass and charge are conserved as always. This species does NOT exist in water, but it is the characteristic anion in liquid ammonia, a water like solvent that will support more powerful bases than does water. Start studying Conjugate Acid-Base Pairs/Lewis definition slides. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The species remaining after a Brønsted-Lowry acid has lost a proton is the conjugate base of the acid. The species formed when a Brønsted-Lowry base gains a proton is the conjugate acid of the base. Thus, an acid-base reaction occurs when a proton is transferred from an acid to a base, with formation of the conjugate base of the reactant acid and formation of the conjugate acid of the reactant base. (e) a species that can accept a pair of electrons. 2. In the Bronsted-Lowry system, a base is defined as: (a) a proton donor. (b) a hydroxide donor. (c) an electron-pair acceptor. (d) a water-former. (e) a proton acceptor. 3. In the equation: HF + H 2 O H 3 O + + F-(a) H 2 O is a base and HF is its conjugate acid. (b) H 2 O is an acid and HF is Conjugate acids and bases are part of the Bronsted-Lowry theory of acids and bases. According to this theory, the species that donates a hydrogen cation or proton in a reaction is a conjugate acid, while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base. The conjugate base may be recognized as an anion. NH3 + H2O <==> H3O+ + NH2-NH2- is therefore the conjugate base of ammonia. HPO4^2- is also amphoteric. It can donate a proton as an acid, or accept a proton to make behave as a base. To have a... Q1. conjugate base --> is the base which forms when NH3 acts as an acid so, for NH3 to act as an acid, it must lose 1 H+ NH3 --> NH2- + H+ then NH2- is the base choose C Q2 note view the full answer
[index] [5762] [1586] [7758] [9831] [1030] [5554] [7547] [6610] [3784] [3273]
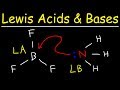
For example, OH− and NH3 are Lewis bases, because they can donate a lone pair of electrons. In the adduct, the Lewis acid and base share an electron pair furnished by the Lewis base. Usually the ... high school chemistry Acid Base Equilibrium Organic Chemistry (Part 1) which side of an acid base reaction is favored HOW TO PREDICT THE EQUILIBRIUM DIRECT... This organic chemistry video tutorial provides a basic introduction into lewis acids and bases. It explains how to predict the products of a lewis acid-base... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... http://leah4sci.com/aminoacids presents: Isoelectric Point of Amino Acids detailed tutorial with time-saving MCAT ShortcutIs your MCAT just around the corner... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... To tell if NaCl (Sodium chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three simple rules along with the neutralization re... How can you figure out which chemical is the Lewis acid and Lewis base in a chemical reaction?Free chemistry help @ www.chemistnate.com
Copyright © 2024 top.realmoneybestgame.xyz